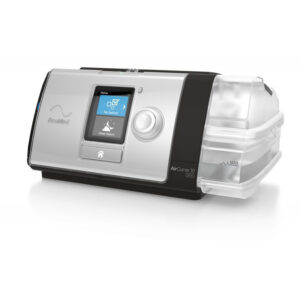
Therapy Device
with remote patient monitoring
Equipment Solution for your body
Healthcare Furniture Needs & Medical Equipment
. Usability and Human Factors Engineering - IEC 62366, FDA Guidance
. Design for Reliability ( DFR ), Design Failure Mode and Effects Analysis (DFMEA ) and Failure reporting, Analysis, and Corrective Actions
. Clinical Evaluation
. CDSCO Registration for Importer and Manufacturer
. Medical device regulations and standards - ISO 14971: 2019, IEC 60601, IEC 60620 and IEC 610000 Series, 21 CFR Part 803 and 820, ISO 13485, IEC
62304, IEC 62366 and European Device Regulation - MDR & IVDR.
. Design for Standard Compliance, Testing and Certification for Medical devices
. Design for Bio-compatibility - ISO 10993
. Cleaning, Disinfection and Sterilization including Clean Rooms
Shop By Categories
-
148 Products
-
54 Products
-
146 Products
-
31 Products
-
34 Products
-
279 Products
-
203 Products
-
95 Products
Shop By Trends
Nareena offers Nebulizer machine with low noise
Featured
Selling
Latest